The development of room temperature sodium ion battery
Jul 12, 2019 Pageview:985
In many electrochemical energy storage systems, lithium ion (Li) (secondary) batteries for its high energy density and long cycle life and other advantages, has gained scale application. However, due to the wide application of lithium resources in industry, using alternative electrochemical energy storage amount is limited, have report estimates that by 2050 there will be up to a quarter of global lithium resources are used for electric cars. For more economic and environmental development with the needs of the energy storage system, sodium (Na) as the object ion secondary battery system has been widely studied. This is based on Na and Li electrode reversible storage and migration mechanism of similarity, ranked sixth in the abundance in the crust and Na element (about 2.6%), at the same time, the water there is a vast resource of Na. Because the Na has larger ionic radius (Na + : 1.02 A, Li + : 0.76 A) and A lower standard electrode potential (about 2.71 Vvs. Na + / Na, about 3.04 Vvs. Li + / Li), and this leads to sodium ion battery energy density is lower than commonly lithium ion batteries. Nevertheless, costs and resources in large-scale energy storage system is the primary consideration, preliminary calculation shows that the future of sodium ion batteries cost about 0.37 yuan/Wh, which is lower than the cost of the lithium ion battery is 0.47 yuan/Wh, and still have to continue to reduce space.
The working principle of sodium ion battery and lithium ion batteries are similar, the same across the electrodes based on Na + in reversible embedded in/out the "rocking-chair batteries" mechanism: in the process of charging, Na +, circuit from the positive emerge after electrolyte embedded negative, and electrons from the positive to the negative movement in the external circuit; Discharge process, on the contrary, the typical lithium ion battery and sodium ion battery structure as shown in figure 1.The key of the sodium ion battery material including anode materials, electrolytes and diaphragm material, etc., this paper mainly discusses the current main is liquid water electrolysis anode materials and organic as well as full battery system based on the research results.
Figure 1 lithium ion batteries (a) and sodium ion battery (b) working principle diagram
A, types and progress of the electrode materials
Sodium ion battery cathode materials mainly include typical lamellar structure and tunnel structure of transition metal oxide material, polyanionic compounds, organic compounds and Prussian blue cathode material; Anode materials mainly include carbon anode materials, metal oxide materials and alloy materials and organic cathode materials.
1. The anode material
Transition metal oxides (TMO2) mainly includes layered and tunnel structure material. Layered classification of transition metal oxides mainly follow Delmas etc. Put forward the structure of the classification, namely according to the different stacking sequence of O, mainly divided into O3, P2 and P3 phase (O3: ABCABC stack;P2: ABBA stack;P3: ABBCCA stack).Sodium ions in tri-prism (P) or octahedron (O) in the interlayer spacing of diffusion, as shown in figure 2.
Figure 2 layer of transition metal oxide structure diagram
As early as 1988, Shacklette on O3, O '3, P2 and P3 phase NaxCoO2 electrochemical properties are studied, found the P2 - NaxCoO2 showed higher energy density and longer cycle life, this makes the P2 - NaxCoO2 has attracted a great deal of research interest. Then, through electrochemical tests in situ XRD and other means such as Delmas of P2 - NaxCoO2 off/embedded mechanism are studied, found that as the change of Na content, appeared on the constant current loop curve of nine different phase transition point, this is mainly due to the different Na space. Considering the toxicity of cobalt (Co) and cost problems, based on other transition metal elements, especially the positive materials of the manganese element has been widely concerned.P2 - NaxMnO2 (0.45 x 0.85 or less or less) with high electrochemical activity. Mr Caballero on P2 - Na0.6 MnO2 research shows that: the material under the voltage range from 2.0 V to 3.8 V, the initial irreversible capacity is 140 mAh/g, but its structure, poor stability, short cycle life. And O3 phase alpha NaMnO2 no significant structural changes in the process of circulation, accordingly, shows good electrochemical performance: in the voltage range from 2.0 V to 3.8 V, the first week of discharge specific capacity as high as 185 mAh/g, circulation after 20 weeks, the specific capacity of 132 mAh/g.In addition, the O3 - NaNiO2 O3 phase and NaCrO2 also has the corresponding electrochemical activity. In a layered transitional metal oxides doped noble metal, such as magnesium (Mg), manganese (Mn), iron (Fe), etc., can enhance the structural stability, inhibition of phase change, for better electrochemical reversibility. Through to the alpha NaFeO2 doping Mn element in frame structure, get the anode material P2 - NaxFe0.5 Mn0.5 O2 (reversible interval of 0.13 x 0.86 or less) or less, when the voltage range of 1.5 ~ 4.3 V (vs. Na + / Na.), reversible electrode is better. Komaba etc. Research has shown that NaNi0.5 Mn0.5 O2 in the current density of 4.8 mA/g, from 2.2 V to 3.8 V (vs. Na + / Na) under the voltage range of the reversible capacity of 105 ~ 125 mAh/g.
Tunnel structure Na0.44 MnO2 (as shown in figure 3 (a)) belongs to the orthogonal crystal system, space group Pbam. As early as 1994, Doeff first reported the tunnel structure Na0.44 MnO2 electrochemical activity. Sauvage, etc. in the water system in the electrolyte of Na0.44 electrochemical performance of MnO2 was studied, the results show that in 2 ~ 3.8 V (vs. Na + / Na) voltage range, as high as 140 mAh/g reversible capacity can be obtained, and the electrode process has experienced six two phase transformation. In full battery system, common anode materials and Na0.44 MnO2 consisting of whole battery system cannot provide enough sodium, it is difficult to obtain and half cell fairly high specific capacity, therefore, need to design a higher sodium content of tunnel type sodium storage material.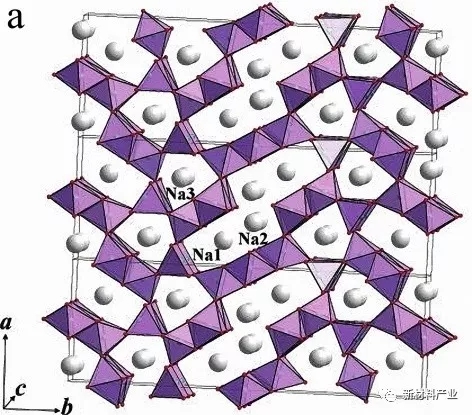
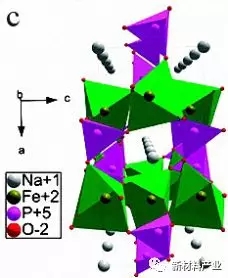
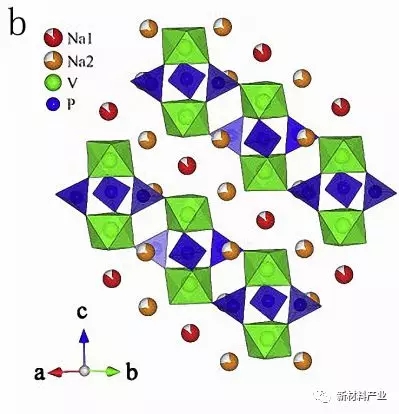
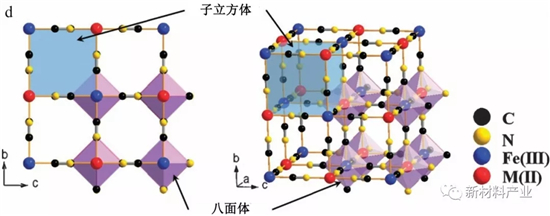
Figure 3 (a) Na0.44 MnO2, (b) Na3V2 (PO4) 3, olivine NaFePO4 (c) and (d) KMIIFeIII (CN) 6 structure diagram
With lithium ion batteries are widely used in the anode material of LiFePO4, polyanionic compounds with open framework structure, strong inductive effect and X - O strong covalent bond (X = phosphorus (P), sulfur (S), silicon (Si), boron (B)), so as the sodium ion battery cathode material has properties of fast ion transport, high working voltage, stable structure, etc.
Fast ion conductor NASICON molecule (NASuperIonicCONductor) general formula is AxMM '(XO4) 3, the molecule is MO6 and XO4 polyhedron Angle of three dimensional network structure, as shown in figure 3 (b). Uebou etc. For the first time Na3V2 (PO4) 3 on the electrochemical properties of sodium ion batteries was reported, then a large number of researchers to Na3V2 (PO4) 3 electrochemical performance is improved. Peridot NaFePO4 with one-dimensional Na + transport channel, as shown in figure 3 (c), as a theory of sodium ion battery cathode material capacity as high as 154 mAh/g. Fluoride phosphate materials for its special storage structure and higher sodium reservoir potential also caused the attention of the researchers, including Na2FePO4 and NaVPO4F because have excellent dynamic performance and is widely studied.
The general formula of Prussian blue (PBAs) molecules to KMIIFeIII (CN) 6 (M = Mn, Fe, Co, Ni, zinc, etc.), such materials common complex belongs to the cubic crystal system, space group for Fm3m, structure there is a lot of alkali ion channels for Na + fast/embedded without structure distortion, as shown in figure 3 (d).In 2012, Lu and other first reported KMIIFeIII (CN) 6 as a stream of sodium ion battery cathode material research, the results show that KFe2 (CN) 6 the reversible capacity of about 100 mAh/g, sodium 2 reservoir potential of 3.5 V and 2.6 V, respectively corresponding to the high spin states and the N bonding Fe3 + / Fe2 + electricity for bonding and C low spin states Fe3 + / Fe2 + power to change. In order to improve the AFe2 (CN) 6 (A = KorNa;0 or less electrochemical performance of A 1) or less, has A number of studies using carbon coated, nano and raising the degree of crystallization method were optimized. In addition, in order to develop green and sustainable energy storage system, abundant organic materials of high capacity, environmental friendly, theory, such as mei tan acid disodium salt (Na2C6O6),) four formic acid dianhydride (PTCDA) and) imide (PTCDI), has become a new alternative to traditional inorganic positive electrode material.
2. The cathode material
As sodium ion battery cathode carbon materials mainly include carbon graphite, soft and hard carbon, etc. Graphite materials for lithium ion battery anode materials, lithium storage performance is good, high reversible capacity (theoretical capacity is 372 mAh/g), however as the sodium ion battery cathode material directly effect is not ideal. Jache confirmed for the first time, such as Na solvents were embedded to embedded in graphite layer in the system, the reversible capacity of graphite anode materials is close to 100 mAh/g, circular 1000 weeks later still has a high capacity retention. Stevensa by glucose decomposition got hard carbon materials, such as for the reversible capacity as high as 300 mah/g. Combined with in situ XRD figure and potential capacity of hard carbon storage mechanism of sodium, they think the high slope area corresponding to Na in parallel (or close to parallel) hard carbon layer between the embedded process, low voltage platform area corresponding to Na in the interlayer of the microporous embedded similar to the adsorption process. Charge and discharge curves of hard carbon materials and sodium storage structure diagram as shown in figure 4.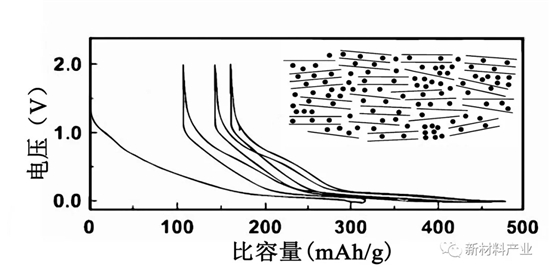
Figure 4 charge-discharge curves of hard carbon materials and store sodium schematic diagram
Metal oxide can be realized by embedding reaction and transformation store sodium, so its as potential sodium ion battery cathode material has caused wide public concern. Xiong, etc. for the first time, the amorphous titanium dioxide (TiO2) nanotubes (TiO2NT) on the electrochemical properties of sodium ion batteries was reported, they found that only a diameter greater than 80 nm TiO2NT show that the electrochemical activity, and specific storage as the cycle number increases gradually. The sodium storage mechanism of the commercial TiO2 nanoparticles studies showed that as the first discharge process, anatase XRD diffraction peak shift to low Angle direction slightly and then gradually disappear, later in the charging process did not return; Na is embedding the Ti4 + to Ti3 +, irreversible part generating Ti0 and NaO2 at the same time. Of Li4Ti5O12 (LTO) research, Yongsheng Hu group reported for the first time the store performance: sodium spinel LTO highest reversible capacity is 155 mAh/g, sodium reservoir potential corresponding to about 0.9 V. Senguttuvan etc. first reported Na2Ti3O7 and superconductive carbon black powder in the sodium ion battery performance, through the analysis of voltage - composition curve, think 0.7 V (vs. Na + / Na) the potential corresponds to black carbon additive reaction, 0.3 V (vs. Na + / Na) the potential corresponding to the two of Na + / embedded (reversible capacity is 200 mAh/g).Other metal oxides, such as: ferroferric oxide (Fe3O4), ferric oxide (Fe2O3), the cobalt oxide (Co3O4), copper oxide and tin oxide (CuO) (SnO2), etc., can store conversion reactions of sodium, compounds such as sodium ion battery cathode material has high theoretical capacity, high performance, cycle the advantages of stable performance.
Metal and metalloid material (such as tin (Sn), antimony (Sb), P, etc.) as a negative electrode materials can be formed Na Me of sodium alloy material in store sodium, and has high theoretical capacity (370 ~ 2000 mAh/g) and sodium lower reservoir potential (less than 1 v).But in the process of the electrode material volume deformation is bigger, does not favor the cycle stability, therefore, studies on anode materials for metal and metalloid currently focused on enhanced circulation stability and Na - Me alloying reaction mechanism two aspects. In addition, a number of studies on anode materials on organic carboxylic acid salts showed that sodium reversible capacity and storage potential of this kind of material is low, for this, including molecular design, surface coating, polymer, improvement methods have been successively research.
Second, the research progress and the whole battery electrolyte materials
As the key part of the battery electrolyte materials, the battery charge transport plays a conduction current role. Good electrolyte should possess the following traits: ionic conductivity; Wide electrochemical window, good thermal stability; Good chemical stability; Security. Based on organic liquid electrolyte of sodium ion battery is the main type of research. Ponrouch systematically studies such as a series of organic electrolyte ion conductivity, viscosity and thermal stability, electrochemical window, etc. Results show that the properties of mixed solvent were superior to single solvent, to the EC: PC (1:1) is the most outstanding, and NaClO4 / NaPF6 - EC: PC (1:1) electrolyte systems exhibit excellent performance in the tests. In electrochemical properties further study found that the battery electrolyte system high reversible capacity (approximately 200 mAh/g), cyclic superior performance (circular 180 weeks).Then, the group in NaClO4 / NaPF6 - EC: on the basis of PC (1:1) continue to study the performance of the ternary electrolyte, the results show that the EC0.45: PC0.45: DMC0.1 electrochemical performance of the best, in Na3V2 (PO4) 2 f3 / hard carbon is available in the whole battery 97 mAh/g specific capacity and stable cycle performance. As a kind of effective lithium ion battery electrolyte additives, fluoro ethylene carbonate (FEC) has also been confirmed as sodium ion batteries of water electrolysis liquid additives can effectively promoted the cell electrochemical performance.
Sodium ion batteries with the deepening of the research of key materials, sodium ions also gradually get the whole battery system. Symmetric full battery anode materials are the same, which makes it has advantages in mass production, cost and safety. Inorganic materials Na2.55 V6O16 · 0.6 H2O (NVO) symmetric full battery specific capacity is about 140 mAh/g, the energy density of about 140 wh/kg, but the cycle performance and rate performance remains to be improved. Organic material 2, 5 - dihydroxy terephthalic acid (Na4DHTPA or Na4C8H2O6) symmetric battery in the first week of the reversible capacity of nearly 200 mAh/g, cycle after 100 weeks capacity remain at a rate of 76%.Embedded in addition, the positive/embedded negative asymmetric type full battery research is quite extensive, but such a full battery, often facing the first week of coulomb low efficiency, poor circulation performance problems. Recently, Yongsheng Hu team preparation by O3 - NaCu1/9 ni2/9 fe1/3 mn1/3 o2 (CNFM) cathode material, hard carbon ball anode materials and assembly of the battery has excellent electrochemical performance: the cycle after 400 weeks, there are still higher than that of the reversible capacity of 200 mAh/g, keep the rate was 71%, the theoretical energy density is 248 wh/kg.
Three, endnotes
For multivariate, new, economic, and the urgent need of large scale energy storage device room temperature sodium ion batteries gradually become a hot research topic, caused wide attention in the world scope, the number of SCI literature published in recent five years rapid increase to more than 300 each year. Sodium ion battery performance and cathode materials, anode materials and electrolyte materials have close relations, so the development of high capacity, long life of electrode materials is the first issue to consider. For the anode material, polyanion materials exhibit excellent stability, and the cost of Prussian blue material has outstanding advantages, by contrast, transition metal oxides are given the highest theoretical specific capacity, therefore each material attracts many researchers. But for anode materials, although a lot of work to investigate the electrochemical properties of metal oxide materials and alloy materials, but for now, only hard carbon material industrialization have to meet the requirements of the reversible specific capacity and cycle life. On the other hand, based on the research of electrode materials is not enough to guarantee the whole battery of the electrochemical properties, development and stability of water electrolysis liquid is the key to high performance of the whole battery build, determines the whole battery cycle life and safety, etc. Several studies have showed less due to the carrier and object function, diffusion of sodium ions in sodium ion battery electrode materials may be more lithium ion, more excellent in principle provides practical future sodium ion battery system on the performance and cycle life is better than that of lithium ion battery, which could provide the future sodium ion batteries in addition to material cost of the core competitive advantage.
Compared with lithium ion battery, first of all, the sodium ion battery cathode material to avoid the consumption of the expensive lithium and more use of the abundant resources of sodium, metal elements such as iron and manganese, and second, sodium ion battery anode materials are all can only use cheap aluminum foil as a collection of fluid, so sodium ion batteries has unique advantages in resources and costs. But the current sodium ion batteries, especially based on organic electrolyte battery system, its basic structure and the traditional non water electrolysis liquid lithium ion battery is the same, the diaphragm, set fluid, the structure of the binder and the battery does not highlight advantages in aspects of cases, the battery on the overall cost, in the short term is beyond support of lithium ion battery has a mature industry chain is not realistic. But on the other hand, the author noticed that lithium ion battery in the early 1990 s industrialization, the cost is very high, but with the development of battery technology, lithium ion battery and continuous increase in performance, cost is declining, makes the current high-performance secondary battery has been able to support electric cars for years to more than 10 years of work. The current mature lithium ion battery industry chain objectively can provide a lot of ready-made material support the development of the sodium ion batteries, strengthen relates to sodium ions in the academia of new electrode materials and system under the premise of exploration, sodium ion battery technology rapid development and mature products can be expected.
The page contains the contents of the machine translation.
- Prev Article: Why do electric cars self-ignite?
- Next Article: Do you know which one is better for electric car, water battery or dry battery?
Leave Message
Hottest Categories
-
Hottest Industry News
-
Latest Industry News









