How to improve the low temperature performance of lithium ion battery electrolyte
Sep 27, 2019 Pageview:1469
Lithium ion batteries as today's most successful chemical energy storage battery one of its footprint in consumer electronics products, not only more open its soil into electric cars.But the performance so outstanding lithium ion battery is very sensitive to temperature, low temperature to cause a decline in lithium ion battery performance and even lead to the lithium ion battery can't use, the low temperature charging more will lead to the production of lithium dendrite, in order to improve the low temperature performance of lithium-ion batteries, the researchers put forward a variety of measures, such as MartaKasprzyk Warsaw university of technology, people put forward the technology of amorphous electrolyte, will the use of the electrolyte temperature to expand to - 60 ℃, Shanghai university professor Xia Yong yao, the ethyl acetate, electrolyte, will further reduce the special material of battery temperature to 75 ℃, of course, not all of the research will focus on the electrolyte, the university of Pennsylvania Guangsheng Zhang and others will design a built-in Ni heating piece of battery, the battery from to 40 ℃ to recover to normal temperature only need 112 s, greatly improve the convenience of the lithium-ion batteries used in low temperature.
Lithium ion battery performance improvement is the key to the electrolyte at low temperature low temperature performance boost, regular commercial lithium ion battery electrolyte viscosity will increase quickly at low temperature, electrical conductivity fell sharply, we use a common commercial lithium ion battery electrolyte LB303 as an example, its ionic conductivity under normal temperature for 10 mS/cm, but in to 40 ℃, its conductivity is sharply dropped to 0.02 mS/cm, serious impact on the low temperature discharge performance of lithium ion battery, therefore improve low temperature performance of lithium-ion batteries is the key to improve the low temperature performance of the electrolyte.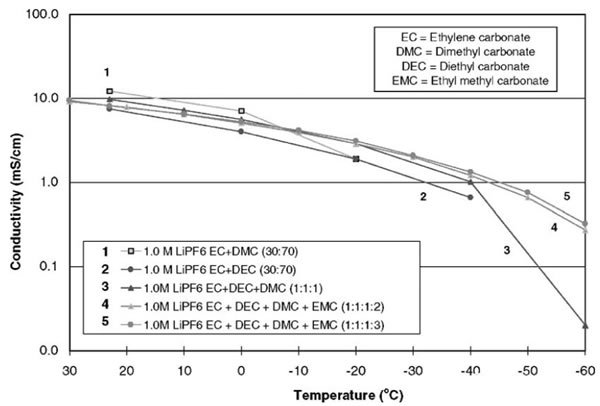
On how to improve low temperature performance of lithium ion battery electrolyte, the United States, of the university of Wisconsin, Milwaukee JanakKafle don't think we need to adding special additives in the electrolyte, only by adjusting the ratio of electrolyte solvent, can significantly improve the low temperature performance of electrolyte.JanakKafle study of cyclic carbonate kind of solvent can reduce the low temperature performance of electrolyte, and straight chain solvent can improve the low temperature performance of electrolyte.
Below shows some common for everyone of the molecular structure of the lithium ion battery solvent and some of the basic physical and chemical index, we can see from the picture of the common solvents EC for rings, EC can help in the negative form better stability of the SEI film, so we hope to add more EC in the electrolyte, but EC high melting point (38 ℃) and the characteristics of high viscosity leads to excessive join EC electrolyte conductivity under low temperature is low, the influence on the performance of the electrolyte at low temperature.Straight chain of solvents, such as DMC, EMC has a relatively low viscosity and good electrochemical stability, so in order to improve low temperature performance of lithium ion battery electrolyte, we usually use a variety of solvent mixture way to improve the low temperature performance of electrolyte, such as the United States at the jet propulsion laboratory M.C.S mart by optimizing the mix of electrolyte solvent, such as the space power supplier SAFT DD size battery (9 ah) using temperature range expanded to 50-40 ℃ (to 40 ℃, C / 10 than energy still can reach 95 wh/kg), so as to enable it to meet the needs of Mars missions.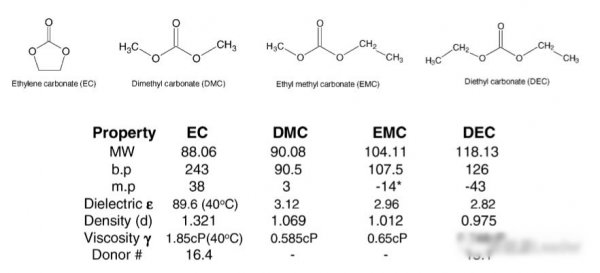
In order to study the different proportion of solvent effect on the properties of the electrolyte temperature, at the university of Wisconsin, Milwaukee JanakKafle design a variety of formulations of electrolyte (shown in the table below, the test battery for NCM111 (0.93 mAh/cm2) positive/graphite anode button cell, the test system is 25 ℃, 1 c, 2 h at low temperature, to achieve heat balance after 5 c battery discharge), from the point of test results, the low temperature of battery discharge capacity depended on the solvent ratio of electrolyte, when more than 40% of the annular solvent, electrolyte at low temperature discharge capacity is significantly reduced.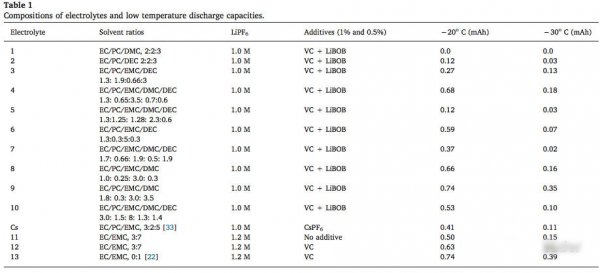
The figure below shows the different EC add ratio electrolyte battery discharge capacity at low temperature, we can very clearly observed from the picture, the discharge capacity of the battery in low temperature increased with the increase of annular solvent EC add ratio decreased significantly.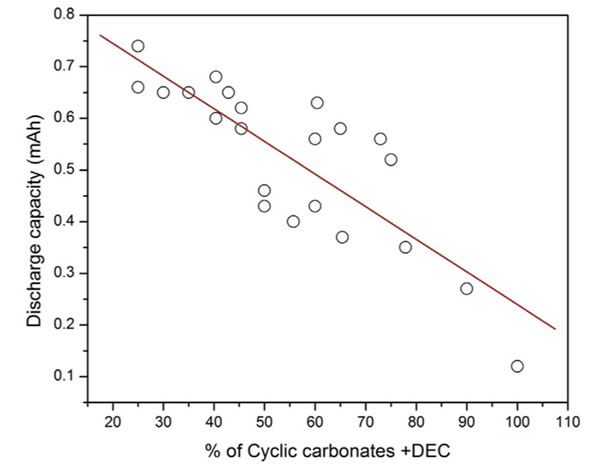
The figure below shows the different proportions of the influence of short chain solvent for the battery discharge capacity at low temperature (due to the EC to add a very small proportion in the whole experiment, is only 20-30%, so the EC for the low temperature performance of the battery is affected, so investigation and) together, we can notice from the picture with the increase of short chain solvent, the low temperature of battery discharge capacity appeared a significant improvement.This actually is not in our regular understanding, because the melting point of DMC and EC at 3 ℃ and 38 ℃ respectively, is not going to significantly reduce the melting point of electrolyte, this suggests that there must be other factors affecting the low temperature properties of the electrolyte.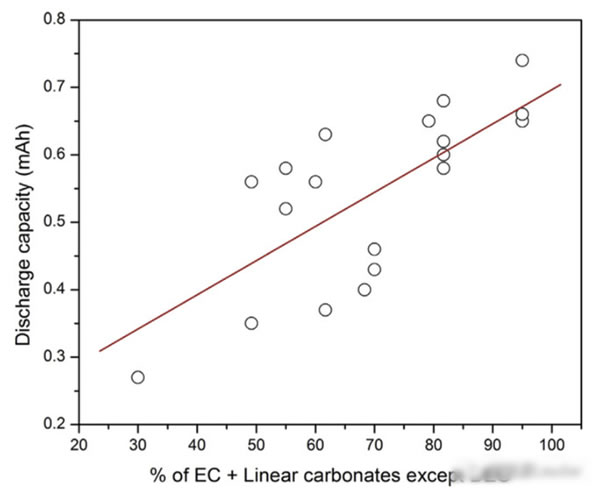
In order to analyze the key factors influencing the electrolyte temperature performance, we need to go back to the first form of this article, we noticed that the electrolyte 11 # can only release under - 20 ℃ electrolyte # 12 to about 80% of capacity, and the only difference between these two kinds of electrolyte with electrolyte increased by 2% in the 12 # VC additive, while 2% of VC additive does not significantly change the conductivity of electrolyte, and the more important is that part of the VC in the process of the battery into reduction decomposition occurs, so we can infer that lead to electrolyte 12 # has better low temperature performance of the key factors are formed better SEI film.
In the following table compares in the electrolyte, 9, 10, and 12 of the formation of the SEI film the proportion of C, O, F and P element, from the table we can notice the different of SEI, the biggest difference in F element in the electrolyte of 9 # in the formation of the SEI film at about 70%, content of F element in the electrolyte 10 # and 12 # F element content of the formation of the SEI film is only 10% and 16%, and we know more LiF means smaller Li + diffusion impedance, which means better discharge performance.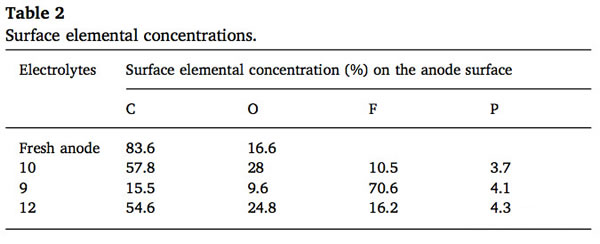
It is easy to find from the above analysis, the focus of the conflict has from the electrolyte conductivity at low temperature, moved to the cathode of the SEI film.SEI is when into lithium ion battery, composition of electrolyte decomposition of porous structure in the cathode surface.SEI porosity and density have significant effect on the performance of the battery, high porosity can stop further reaction of the electrolyte in the cathode surface, while the density is too high to Li + diffusion in significant obstacles.The following table shows the different form of SEI film in different electrolyte 25 ℃ and 20 ℃ under the impedance of the fitting results, from the table we noticed that the temperature falls ohmic resistance Rs change is relatively small, and Li + in the SEI film diffusion resistance R and charge exchange impedance Rcte there had very big change, this suggests that the lower the electrolyte ion conductivity is not the main cause of the low temperature performance battery, really led to the decrease of the battery low temperature performance of the key factors is the increase of interfacial diffusion and charge exchange impedance.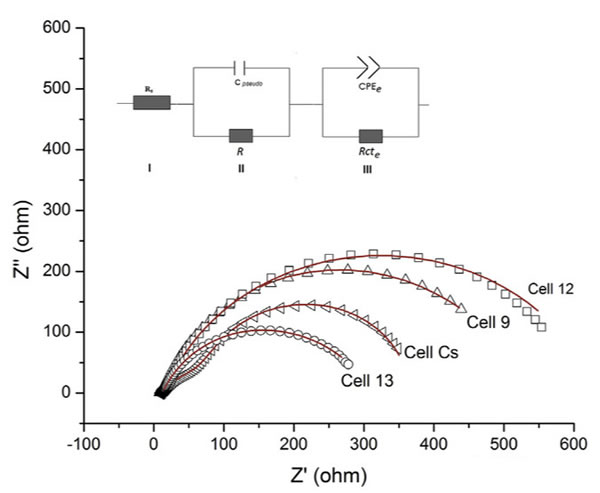
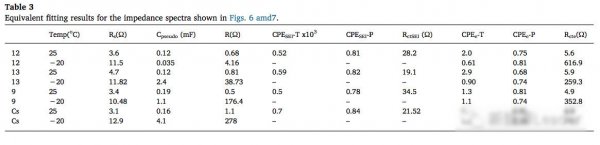
It is not hard to see through the above analysis, the low temperature conductivity of the electrolyte for lithium ion batteries the influence of low temperature performance was not so big, the and the cathode of the composition and structure of the SEI film for low temperature performance of the battery are much more important, the influence of good SEI film should contain more LiF, thus reducing the Li + diffusion impedance in the SEI film.In general more chain solvent, such as EMC and DMC, less ring solvents, such as EC can effectively improve the low temperature performance of lithium-ion batteries, but in order to form a better stable SEI film, we still need to add a small amount of EC and PC.
The page contains the contents of the machine translation.
Leave Message
Hottest Categories
-
Hottest Industry News
-
Latest Industry News









