-
1) Overview of Lithium Cobalt Oxide Batteries+
- 1.How lithium cobalt oxide battery works
- 2.Lithium Cobalt Oxide Battery Reactions
- 3.Lithium Cobalt Oxide Battery Electrolyte
-
2) Advantages and Disadvantages of Lithium Cobalt Oxide Batteries
-
3) Properties of Lithium Cobalt Oxide Anode Material
-
4) Application of Lithium Cobalt Oxide Battery
-
5) Comparison of anode Material Properties of Lithium Battery
Lithium Cobalt Oxide (LCO) Battery - LiCoO2 Manufacturer
Mar 23, 2021 Pageview:8875
1) Overview of Lithium Cobalt Oxide Batteries
Cobalt acid lithium battery has high discharge platform, high specific capacity, stable product performance, and good cycle performance, but its safety performance is poor. The cost is very high, it is mainly used for medium and small type batteries, which is widely used in mobile phones, portable computers, cameras, camera, electric cars, solar energy storage, UPS power supply, medical equipment, space and other fields. The nominal voltage is 3.7V.
Lithium cobalt oxide, LiCoO2 (LCO), is an inorganic compound commonly used as a anode material for lithium ion batteries.
LiCoO2 basically uses liquid phase synthesis process of lithium ion secondary battery cathode material with layered structure in the current commercial lithium ion battery (cobalt acid lithium). It adopts polyvinyl alcohol (PVA) or polyethylene glycol (PEG) aqueous solution as solvent. Lithium and cobalt salt dissolve in PVA or PEG solution, mixing the solution after heating, then they will concentrate into gel formation. After heating and decomposition, calcine the gel formation at high temperature, sinter, grind and sieve, then you can get the cobalt acid lithium powder.
1.How lithium cobalt oxide battery works
Lithium cobalt oxide battery mainly relies on the intercalation and disintercalation of lithium ions between anode and cathode to realize the storage and release of energy.
While charging, at the external electric field, lithium elements in the LiCoO2 molecules of the anode material become lithium ion with positive charge, which moves from the anode to cathode. After chemical reaction with the carbon atoms of the cathode, LiC6 is formed, which is stably embedded into the layered graphite cathode.
When discharging, on the contrary, in the internal electric field turns, Li+ breaks away from the cathode, goes along the direction of the electric field, returns to the anode, and then becomes LiCoO2. This process called a "rocking chair battery". The more lithium ions involved in back-and-forth embedding and disembedding activities, the more energy the battery can store.
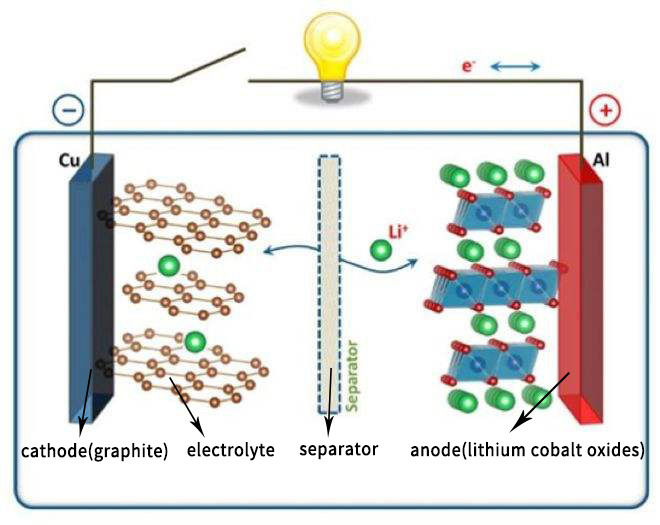
How lithium cobalt oxide battery works
2.Lithium Cobalt Oxide Battery Reactions
What happens when you charge it,
Anode: LiCoO2 = Li1-xCoO2+xLi++xe-
Cathode: 6C+xLi++xe- = LixC6
What happens when you discharge
Anode: Li1-xCoO2+xLi++xe-=LiCoO2
Cathode: LixC6=6C+xLi++xe-
3.Lithium Cobalt Oxide Battery Electrolyte
Composition of Lithium Cobalt Oxide Battery Electrolyte
Solvent: cyclic carbonate (PC, EC); Chain carbonates (DEC, DMC, EMC); Carboxylic esters (MF, MA, EA, MA, MP, etc.); (for dissolving lithium salts);
Lithium salts: LiPF6, LiClO4, LiBF4, LiAsF6, etc.
Additives: film forming additives, conductive additives, flame retardant additives, overcharge protection additives, additives to control H2O and HF in electrolyte, additives to improve low temperature performance, multi-functional additives.
Requirements of Lithium Cobalt Oxide Battery Electrolyte
High ionic conductivity is generally 1x10-3~2x10-2 S/cm;
High thermal and chemical stability: no separation in a wide voltage range;
A wide electrochemical window keeps the electrochemical properties stable in a wide voltage range.
Good compatibility with other parts of the battery, such as electrode material, electrode collector and separator;
It is safe, non-toxic and pollution-free.
2) Advantages and Disadvantages of Lithium Cobalt Oxide Batteries
Among all anode materials of lithium battery, LCO has the largest tap density (2.8g/cm3) and compaction density (4.3 g/cm3), which makes it have advantages in the application of battery field with strict requirements on battery volume. In addition, LCO has better cycling performance, low temperature performance and rate capability than existing ternary materials. So far, it is still the non-cobalt material that is used as anode materials in some 3C batteries, low temperature and high rate batteries.
LCO also has its own shortcomings, the first of which is the resource. The total cobalt resources in the world do not exceed 7.1 million tons (USGS/2016) with an annual output of no more than 120,000 tons and a reserves/production ratio is 57 years. There may be a bright future in the early days of 3C lithium-ion, but the rise of power and storage means that cobalt will soon lag behind.
The second issue is safety. The safety performance of large-capacity LCO batteries is not optimistic, especially in the condition of full charge extrusion, overheating or overcharging. LCO batteries will tend to explode under these circumstances. Even if using a high-safety lithium titanate as the negative electrode of cobalt titanium battery, LCO will explode violently when overcharged and squeezed.
Advantage of Using Lithium Cobalt Oxide As Anode Material
Lithium cobalt oxide battery has stable cell structure because of lithium cobalt oxide.
Lithium cobalt oxide has a higher capacity than other anode materials.
The comprehensive properties of lithium cobalt oxide are better than other anode materials.
Lithium cobalt oxide processing is more convenient.
The consistency of lithium cobalt oxide is good after processing.
The discharge rate of lithium cobalt oxide is high.
Lithium cobalt oxide has high power.
Lithium cobalt oxide battery has good market prospects.
Disadvantages of Using Lithium Cobalt Oxide As Anode Materials
Cobalt ore is scarce and the domestic storage of high-quality cobalt ore is limited.
The price of cobalt ore is high. By the middle of 2018, the price of cobalt ore has reached 700,000 yuan/ton.
It has small cycle times. Currently, the cycle of lithium cobalt oxide can only reaches 500 times, but compared with that of lithium titanate, which can reach 20,000 times, lithium cobalt oxide has lower cycle times as anode materials.
It is not friendly to the environment. Currently, cobalt belongs to heavy metal, which has a certain corrosive effect on soil
The safety performance of lithium cobalt oxide is poor.
3) Properties of Lithium Cobalt Oxide Anode Material
Lithium cobalt oxide performs well in terms of high specific energy, but not so good in power characteristics, safety and cycle life.
| Lithium cobalt oxide: LiCoO2 cathode (about 60% Co), graphite anode | |
| voltage | "Nominal value is 3.60V; Typical operating range is 3.0-4.2v/battery" |
| specific energy(capacity) | 150-200wh /kg, special battery provides 240Wh /kg. |
| Charge(C rate) | "0.7-1C, charge to 4.20V (most batteries); Typical charging time: 3 hours; Charging current above 1C will shorten battery life." |
| Discharge(C rate) | "1C, discharge cut-off voltage is 2.50V Discharge current above 1C will shorten battery life." |
| cycling life | 500-1000, depending on discharge depth, load and temperature |
| thermal runaway | 150 ° c (302°F). Full charge can easily lead to thermal runaway |
| application | Mobile phones, tablets, laptops, cameras, instruments, etc. |
| note | It has very high specific energy, but limited specific power. Cobalt is expensive, and used in energy battery. Its market share is stable. |
Microelement Content in Lithium Cobaltate Anode Material
| micro elements in lithium cobaltate | Ni | Mn | Fe | Ca | Na |
| content(%) | 0.05 | 0.01 | 0.02 | 0.03 | 0.01 |
4) Application of Lithium Cobalt Oxide Battery
Lithium cobaltate is the first generation of commercial anode materials. In decades of development, it can be considered as the most mature anode materials of lithium ion battery.
Lithium cobalt oxide is still the best choice for small lithium batteries. At present, as for 3C products, most of them still use lithium cobaltate instead of ternary materials with higher specific capacity, because the compaction density of lithium cobaltate is higher than that of ternary materials, that is, more lithium cobaltate can be contained in unit volume. Lithium cobalt oxide plays an important role in small size batteries that concentrate on volume density.
As the technology development and market changing, the requirement of lithium cobaltate battery is also rising slowly. It has been used in electronic equipment such as tablet and mobile phone, and also expand to different domain in recent years. Due to its good stability and consistency, high energy density and other advantages, lithium cobalt oxide battery has been gradually applied to medical equipment, instrumentation, emergency backup, special communication equipment, etc.
5) Comparison of anode Material Properties of Lithium Battery
Anode material is one of the key materials that determine the performance of lithium ion batteries, and is also the main source of lithium ion in the current commercial lithium ion batteries. Its performance and price have a great impact on lithium ion batteries. At present, the anode materials has been developed and applied, mainly including lithium cobalt oxide (LCO), lithium manganese oxide (LMO), ternary materials such as lithium nickel cobalt manganese oxide (NCM), lithium nickel cobalt aluminate (NCA), lithium iron phosphate (LFP) and lithium titanate (LTO).
Performance Comparison of Several Commercial Anode Materials
| Item | lithium cobalt oxide | lithium manganese oxide | lithium nickel cobalt manganese oxide | lithium nickel cobalt aluminate | lithium iron phosphate | lithium titanate |
| chemical formula | LiCoO2 | LiMn2O4 | LiNiCoMnO2 | LiNiCoAlO2 | LiFePO4 | Li2TiO3 |
| theoretical capacity (mAh/g) | 274 | 148 | 275 | 275 | 170 | 175 |
| actual capacity(mAh/g) | 140 | 120 | 160~220 | 180 | 150 | 160 |
| tap density (g/cm3) | 2.8 | 2.2 | 2.6 | 2.6 | 1 | 1.68 |
| compaction density (g/cm3) | 4.2 | 3 | 3.6 | 3.6 | 2.2 | 2.43 |
| voltage platform (V) | 3.7 | 4 | 3.5 | 3.5 | 3.3 | 2.4 |
| cycling life | better | worse | ordinary | ordinary | good | better |
| transition metal | scarce | abundant | more abundant | more abundant | abundant | shortage |
| cost of raw material | more expensive | cheap | expensive | expensive | cheap | expensive |
| environmental protection | contain cobalt | non-toxic | contain nickel and cobalt | contain nickel and cobalt | non-toxic | non-toxic |
| safety performance | bad | good | better | better | best | better |
The diagram below compares the specific energies of lead, nickel and lithium systems. Although lithium aluminum (NCA) is the winner by storing more capacity than other systems, it is only suitable for power usage in certain scenarios. Lithium manganate (LMO) and lithium phosphate (LFP) are superior in specific power and thermal stability. Lithium titanate (LTO) may have a lower capacity, but it has the longest life than other batteries and has the best low-temperature performance.
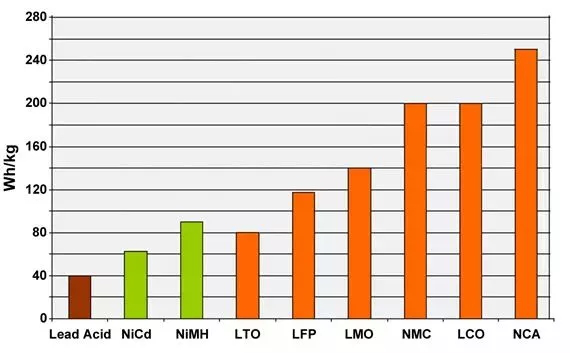
Typical specific energy of lead, nickel and lithium - based batteries
NCA has the highest specific energy; However, lithium manganate and lithium iron phosphate are superior in power and thermal stability. Lithium titanate has the best service life.
- Prev Article: Lithium Battery Shipping Boxes
- Next Article: What Is Difference Between Alkaline And Lithium Batteries
Leave Message
Hottest Categories
-
Hottest Industry News
-
Latest Industry News












