-
1) Overview of Lithium Manganate Oxide Batteries
-
2) LMO Battery Cathode
-
3) LMO Battery Composition
-
4) How Lithium Manganate Oxide Batteries Work
-
5) Advantages and Disadvantages of Lithium Manganate Oxide Battery
-
6) The Characteristics of Lithium Manganate Oxide Battery
-
7) LMO Battery Applications
-
8) LMO vs NMC Battery
-
9) Comparison of Anode Material Properties of Mainstream Lithium Battery
Lithium Manganese Oxide (LMO) Battery - LiMn2O4 Manufacturer
Mar 23, 2021 Pageview:7293
1) Overview of Lithium Manganate Oxide Batteries
Lithium manganate oxide battery refers to the battery that uses lithium manganate oxide as an anode material. Its nominal voltage is 3.7V. It is the mainstream power battery at present. This kind of battery has ordinary energy density and cycling life. It has environmental protection and no patent limitation. But lithium manganate oxide is not very stable, easy to decompose gas, may lead to swell, and its high-temperature performance is poor.
Lithium manganate oxide, whose chemical formula is LiMn2O4 (LCM), is one of the promising lithium ion anode materials. Compared with traditional anode materials such as lithium cobalt oxides, lithium manganate oxide has rich resources, low cost, no pollution, good safety and nice rate capability. It is an ideal anode material for power battery.
Lithium manganate oxide tends to be spinel type. LiMn2O4 spinel manganese acid lithium is the first anode material with 3D Li ion channel that made by Hunter in 1981. So far, it has been attracted attention from many scholars and researchers both at home and abroad. As an electrode material, it has low price and high potential, environment friendly and good safety performance, and is the most promising anode materials of new generation of lithium ion battery to replace LiCoO2.
2) LMO Battery Cathode
The main components of lithium manganate oxide are spinel lithium manganate oxide and layered structure lithium manganate oxide. The model of spinel structure lithium manganate oxide belongs to cubic system, which is a kind of Fd3m space group. At present, the high-capacity lithium manganate oxide anode material has a reasonable structure. Lithium ions can be easily and reversibly detached from the spinel lattice, because the structure is relatively secure, there is no risk of structural collapse, and the safety of the product is guaranteed.
1. Layered structure LiMnO2 has theoretical capacity of 285mA·h/g and voltage platform of 4V. The layered structure is difficult to synthesize and is unstable. It is easy to generate spinel structure Li2Mn2O4, which leads to the decline of voltage platform, poor stability and irreversible capacity decay.
2. High-pressure spinel LiMn2O4 has theoretical capacity of 148mA·h/g and voltage platform of 4.15. Its high-temperature performance is poor, and the capacity will have serious attenuation above 55℃. It is easy to produce spinel structure Li2Mn2O4, which leads to voltage platform decline, poor stability, irreversible capacity attenuation, etc. This is the lithium manganate oxide currently used in industry.
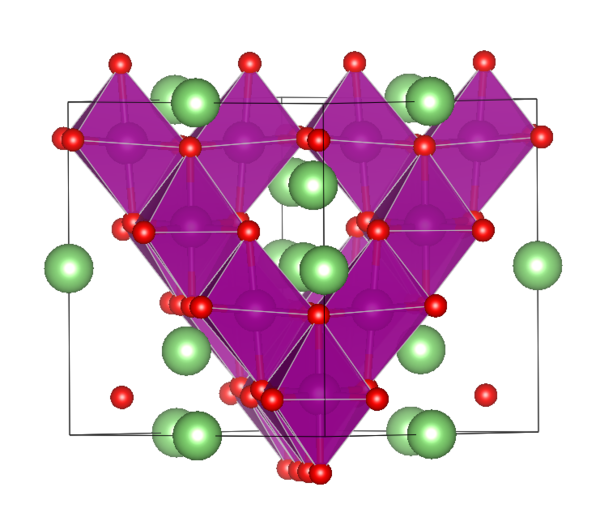
Structure Diagram of Spinel Lithium manganate oxide
3.Spinel Li2Mn2O4 has electric depression of 3V, low capacity and poor circulation. People are studying how to avoid these issues.
3) LMO Battery Composition
Lithium manganate oxide battery is a lithium ion battery that uses lithium manganate oxide as anode, graphite as cathode, and electrolyte with LiPF6 organic solution. Its nominal voltage is 3.7V. The structure of lithium manganate oxide battery packed in aluminum shell is shown in the following figure:
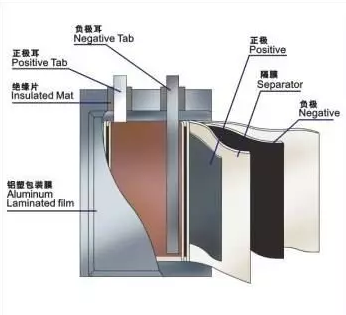
Anode structure: LiMn2O4(lithium manganate oxide)+ conductive agent (acetylene black)+ adhesive (PVDF)+ collector (aluminum foil) anode
Cathode structure: graphite + conductive agent (acetylene black)+ adhesive (PVDF)+ collector (copper foil) cathode
Separator: a special composite membrane
Case: divided into steel case and aluminum case
Organic electrolyte
Top cover and bottom cover
4) How Lithium Manganate Oxide Batteries Work
When a lithium manganate oxide battery is charged, lithium ions in the anode detach from the lattice, pass through the electrolyte to the cathode surface and embed into the graphite layer. When you discharge, the process is reversed. In charging and discharging process, lithium ions go between the anode and cathode, which is also known as the "rocking chair" battery.
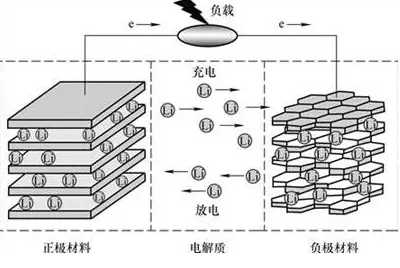
Schematic Diagram of the Working Principle of LiMn2O4
Charging Process
When the battery is charged by the power source, the electron on the anode runs to the cathode from the external circuit. The lithium ion (Li+) goes into the electrolyte from the anode, passes through the small winding hole in the separator, reaches the cathode, and combines with the electrons that have already come over.
The reaction on the anode: LiMn2O4==Li1-xMn2O4+Xli++Xe(electron)
The reaction on the cathode: 6C+XLi+Xe==LixC6
Discharging Process
When the battery discharges, the electron on the cathode runs from the external circuit to the anode, and the lithium ion goes into the electrolyte from the cathode, passes through the small winding hole in the separator runs to the anode, and combines with the electrons that have already come over.
The reaction on the anode Li1-xMn2O4+xli++xe(electron)==LiMn2O4
The reaction on the cathode LixC6==6C+xLi+xe
5) Advantages and Disadvantages of Lithium Manganate Oxide Battery
Lithium manganate oxide has the advantages of good rate capability, easy preparation and low cost. The disadvantage is that the dissolution of manganese leads to poor high-temperature performance and cycling performance. The high-temperature performance and cycling performance are greatly improved by doping aluminum and sintering granulation, which can basically meet the actual use. In general, lithium manganate oxide battery has low cost, good stability and wonderful low-temperature performance, but its high-temperature performance is poor, and it has slightly faster attenuation.
The anode materials have low cost, good safety, and nice low-temperature performance, but the material itself is not so stable, and easy to decompose to produce gas, so they tend to be mixed with other materials, in order to reduce the cost of batteries. However, the attenuation of cycling life goes quickly, and prone to bulge. Its high-temperature performance is poor, and cycling life is relatively short. It is mainly used for large and medium size batteries, power battery. The nominal voltage is 3.7 V.
6) The Characteristics of Lithium Manganate Oxide Battery
"Lithium manganate oxide: LiMn2O4 cathode, graphite anode Abbreviation: LMO or Li-Mn (spinel structure), since 1996" | |
| voltage | 3.70V (3.80V) nominal value; Typical operating range 3.0-4.2V/battery |
| specific energy(capacity) | 100-150Wh/kg |
| charge(C rate) | Typical value is 0.7-1C, maximum is 3C, charge to 4.20V (most batteries) |
| discharge(C rate) | 1 C. Some batteries can reach 10C, 30C pulse (5s), 2.50V cut-off |
| cycling life | 300-700 (related to discharge depth and temperature) |
| thermal runaway | Typical values are 250°C (482°F). High charge promotes thermal runaway |
| tap density(g/cm3) | 2.8~3.0 |
| specific surface area(m2/g) | 0.4~0.6 |
| voltage platform(V) | 4 |
| transition metal | abundant |
| material cost | cheap |
| environmental protection | non-toxic |
| safety performance | good |
| application | Power tools, medical equipment, electric powertrain |
7) LMO Battery Applications
Lithium manganate oxide is one of the promising lithium ion anode materials. Compared with lithium cobalt oxide and other traditional anode materials, lithium manganate oxide has advantages of rich resources, low cost, no pollution, good safety and nice rate capability. It is an ideal anode material for power battery. Therefore, lithium battery electrode materials, especially the new-generation lithium manganate oxide anode materials, have great potential in the electric vehicle power battery market. Due to the low price and rich resources of lithium manganate oxide, it is easier to be produced than lithium cobalt oxides and lithium nickelate, which will make lithium ion battery enter a new era of development.
Because lithium manganate oxide material has such distinct characteristics, people will make use of its advantages and avoid its disadvantages. Therefore, lithium manganate oxide battery is applied in different fields, which are usually called class A and class B applications.
Class A refers to the power battery, which focuses on safety and recycling performance. It is required to have a reversible capacity of 100~115mAh/g and maintain 80% capacity after 500 cycles.
Class B is mainly used for consumer electronics. It is characterized by high capacity. Generally, it is required to have a reversible capacity of 120 mAh/g and maintain 60% capacity after 300~500 cycles. The different performance of these two types of batteries is realized in the production process.
8) LMO vs NMC Battery
LMO Battery(LiMn2O4)
Lithium manganate oxide battery is a battery which uses lithium manganate oxide material in anode. The nominal voltage of lithium manganate oxide battery is 2.5~4.2V. Lithium manganate oxide battery is widely used for its low cost and good safety.
lithium manganese oxide battery has low cost, good safety, and nice low-temperature performance, but the material itself is not so stable, and easy to decompose and produce gas, so it tend to be used with other materials, in order to reduce the cost of batteries. However, it has quick attenuation of cycling life, and is prone to bulge with poor high-temperature performance and relatively short life. It is mainly used for large and medium size batteries and power battery. Its nominal voltage is3.7 V.
NMC(LiNiMnCoO2)
The ternary lithium battery is a lithium ion battery which uses lithium NiCoMnO2 or NCA for anode. The raw materials of ternary composite anode material are nickel salt, cobalt salt and manganese salt. The proportion can be adjusted according to the actual needs. Battery with ternary materials as anode has relatively better safety than lithium cobalt oxide battery.
NCM is one of the key materials of lithium ion battery. NCM replaces over two thirds of cobalt by cheaper nickel and manganese, so it has obvious cost advantage. Compared with other lithium ion anode materials, including lithium manganate and lithium iron phosphate, NCM and lithium cobalt oxides has similar electrochemical performance and processing performance, which makes NCM materials be the new battery materials and gradually replace lithium cobalt oxides, and become the trend of the new generation of lithium ion battery materials.
Compare LMO and NMC battery
LMO has no precious metal and its price is relatively low; NCM is expensive affecting by cobalt enterprises (high nickel materials reduce BOM costs, but increases process costs).
LMO voltage platform is high, 3.75~ 3.8V; NCM voltage platform is low, 3.6~ 3.7V.
LMO with spinel structure is relatively safe; NCM with layered structure is easy to mix lithium and nickel, and has poor safety (the higher Ni%, the worse it is).
As for the most critical gram volume, LMO is lower, while NCM is higher.
LMO high-temperature performance is particularly poor, so the industrial method is LMO doping ternary. Typical percentage is LMO: NCM=7:3 (low cost, e-bike), 5:5 or 3:7 (cargo van)
After adding LFP, the overall cost: pure LMO < LFP≤LMO mixing NCM < NCM
Average life span
The characteristics of lithium manganate are more applicable to: (1) cost limitation; (2) energy density has a certain requirement; (3) safety requirements are not so serious in the field, such as electric bicycles, low speed car, etc.
9) Comparison of Anode Material Properties of Mainstream Lithium Battery
Anode material is one of the key materials that determine the performance of lithium ion batteries, and is also the main source of lithium ion in the current commercial lithium ion batteries. Its performance and price have a great impact on lithium ion batteries. At present, the anode materials developed and applied mainly include lithium cobalt oxide (LCO), lithium manganese oxide (LMO), ternary materials such as lithium nickel cobalt manganese oxide (NCM), lithium nickel cobalt aluminate (NCA), lithium iron phosphate (LFP) and lithium titanate (LTO).
Performance Comparison of Several Commercial Anode Materials
| item | LCO | LMO | NCM | NCA | LFP | LTO |
| chemical formula | LiCoO2 | LiMn2O4 | LiNiCoMnO2 | LiNiCoAlO2 | LiFePO4 | Li2TiO3 |
| theoretical capacity (mAh/g) | 274 | 148 | 275 | 275 | 170 | 175 |
| actual capacity (mAh/g) | 140 | 120 | 160~220 | 180 | 150 | 160 |
| tap density (g/cm3) | 2.8 | 2.2 | 2.6 | 2.6 | 1 | 1.68 |
| compaction density (g/cm3) | 4.2 | 3 | 3.6 | 3.6 | 2.2 | 2.43 |
| voltage platform (V) | 3.7 | 4 | 3.5 | 3.5 | 3.3 | 2.4 |
| cycling life | better | worse | ordinary | ordinary | best | better |
| transition metal | scarce | most abundant | more abundant | more abundant | most abundant | shortage |
| material costs | more expensive | cheap | expensive | expensive | cheap | expensive |
| environmental protection | contain cobalt | non-toxic | contain nickel and cobalt | contain nickel and cobalt | non-toxic | non-toxic |
| safety performance | bad | good | better | better | best | better |
The diagram below compares the specific energies of lead, nickel and lithium systems. Although lithium aluminum (NCA) is the winner by storing more capacity than other systems, it is only suitable for power usage in certain scenarios. Lithium manganate (LMO) and lithium phosphate (LFP) are superior in specific power and thermal stability. Lithium titanate (LTO) may have a lower capacity, but it has the longest life than other batteries and has the best low-temperature performance.
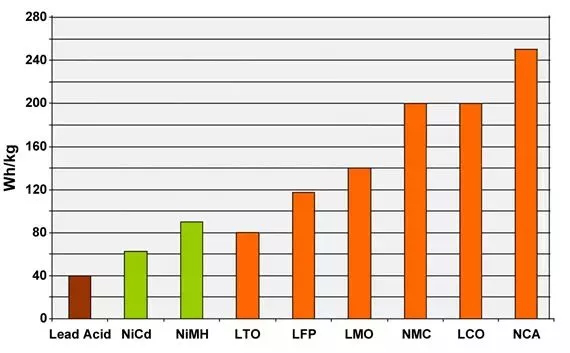
Typical specific energy of lead, nickel and lithium - based batteries
NCA has the highest specific energy; However, lithium manganate and lithium iron phosphate are superior in power and thermal stability. Lithium titanate has the best service life.
- Prev Article: Magnets and Lithium-ion Batteries Attention
- Next Article: LiPo Battery Life Expectancy Analysis
Leave Message
Hottest Categories
-
Hottest Industry News
-
Latest Industry News












