What is the compromise between negative delithium voltage and reversible capacity?
Jun 03, 2019 Pageview:615
Strictly speaking, the compromise between the mass ratio and the voltage does not include lithium metals. Lithium metal operating voltage 0V, theoretical capacity 3862 mAh/g, actual capacity is determined by the utilization rate of active substances.
Why compromise? In general, the current negative electrode candidate(excluding metallic lithium) has a basic feature that the larger the capacity of the active electrode material, the higher the delithium voltage platform(or average value). For example, the average delithium potential of graphite carbon materials is 0.15 V. The actual capacity is 350 mAh/g; The average delithium potential of the Sn negative electrode is 0.5 V and the theoretical capacity is 990 mAh/g; The average delithium potential of Si negative electrode is 0.45 V and the theoretical capacity is 4200mAh/g. The actual available capacity of Sn and Si is still uncertain and is ultimately determined by the conditions of use.
The energy density and specific characteristics of the battery are determined by the product of the average operating voltage(positive and negative voltage difference) and the mass specific capacity(or volumetric capacity). When replacing carbon-based materials with Si or Sn, Whether the increase in battery specific capacity can make up for the reduction of battery operating voltage is an important factor to be considered. A simple example shows that if lithium iron phosphate is positive, its operating voltage is measured in 3.45 V and its capacity is measured in 160mAh/g; When matched with graphite carbon(in 0.15 V, 350mAh/g actual capacity), lithium iron 1G phosphate matches 0.457 g graphite carbon, with a total battery theoretical energy ratio of(3.45-0 .15) V * 160mAh/1 .457 g = 362.3 mWh / G.
Lithium 1G iron phosphate matches the Si negative electrode, which is calculated according to 0.45 V and 4200 mAh/g. The matched total battery specific energy is(3.45-0 .45) V * 160mAh/1.038 g = 462 mWh/g. Obviously, after replacing the graphite carbon negative with the Si negative electrode, the increase in capacity can make up for the impact of the decrease in battery voltage caused by the increase in the delithium voltage. Of course, this is a theoretical consideration because the actual capacity of the Si negative pole can not reach its theoretical value. Assuming that the actual capacity of the Si negative electrode is P, to satisfy the condition that the total battery specific energy is greater than 362.3 mWh / g, the relationship satisfied is
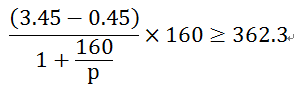
Of course, the above formula is still a little simplified, without considering the relationship between Si negative voltage and capacity, but it can explain the purpose we are currently discussing. The calculation of the available P is at least 492.5 mAh/g. In other words, the actual capacity of the Si negative pole is greater than 492.5 mAh/g to ensure that the mass ratio of the whole battery does not deteriorate(compared with the lithium ferrophosphate / graphite carbon system). The development of high-capacity C-Si composite negative materials can also draw on the above discussion. Roughly speaking, the capacity of the Si part of the C-Si composite negative electrode in practical applications can not be less than 492.5 mAh/g, otherwise there is no point.
This is the compromise between the increase of negative electrode delithium voltage and the increase of reversible capacity. Because there is a compromise relationship between these two parameters, lithium titanate and nitride are directly ignored here because the voltage platforms of the two are really high and can not be tolerated.
The page contains the contents of the machine translation.
- Prev Article: Car and home exit punk approach electric car rental share prospects are still promising
- Next Article: Watma is deep in debt
Leave Message
Hottest Categories
-
Hottest Industry News
-
Latest Industry News











